Scientists at the Academy needed a solid understanding of the biology and physics involved before they could design a way to safely bring fish up from the twilight zone. The very core of the problem is that fish can’t survive a rapid change in pressure.
In general terms, pressure is force distributed over an area:
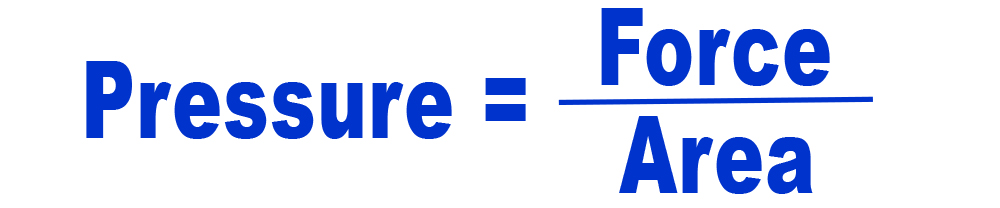
For example, we experience pressure from the air all around us. Air is made up of many molecules, mainly nitrogen and oxygen. These molecules are constantly bumping into us from all different directions. The force from these molecules bumping against us is what creates air pressure.
Air Pressure Activity
Create a change in the air pressure around you using a garbage bag and vacuum! Capture the process on video or with photos and share them with us. Or, draw an illustration or make a concept map that explains what happened to share. You can submit your work on our website here or via Vine or Instagram and tweet it out to @KQEDEdspace with hashtag #kqedpressure. Check out what others have submitted on our website .
View the activity.
We don’t tend to notice air pressure because it remains relatively constant when we stay at the same elevation. However, air pressure decreases as we move from sea level to the top of a mountain. There are fewer air molecules bumping against you the higher up you go, so the air pressure is lower. You may have felt the effects of this pressure change when flying in an airplane or driving to the top of a mountain. The pressure change causes your ears to “pop.”
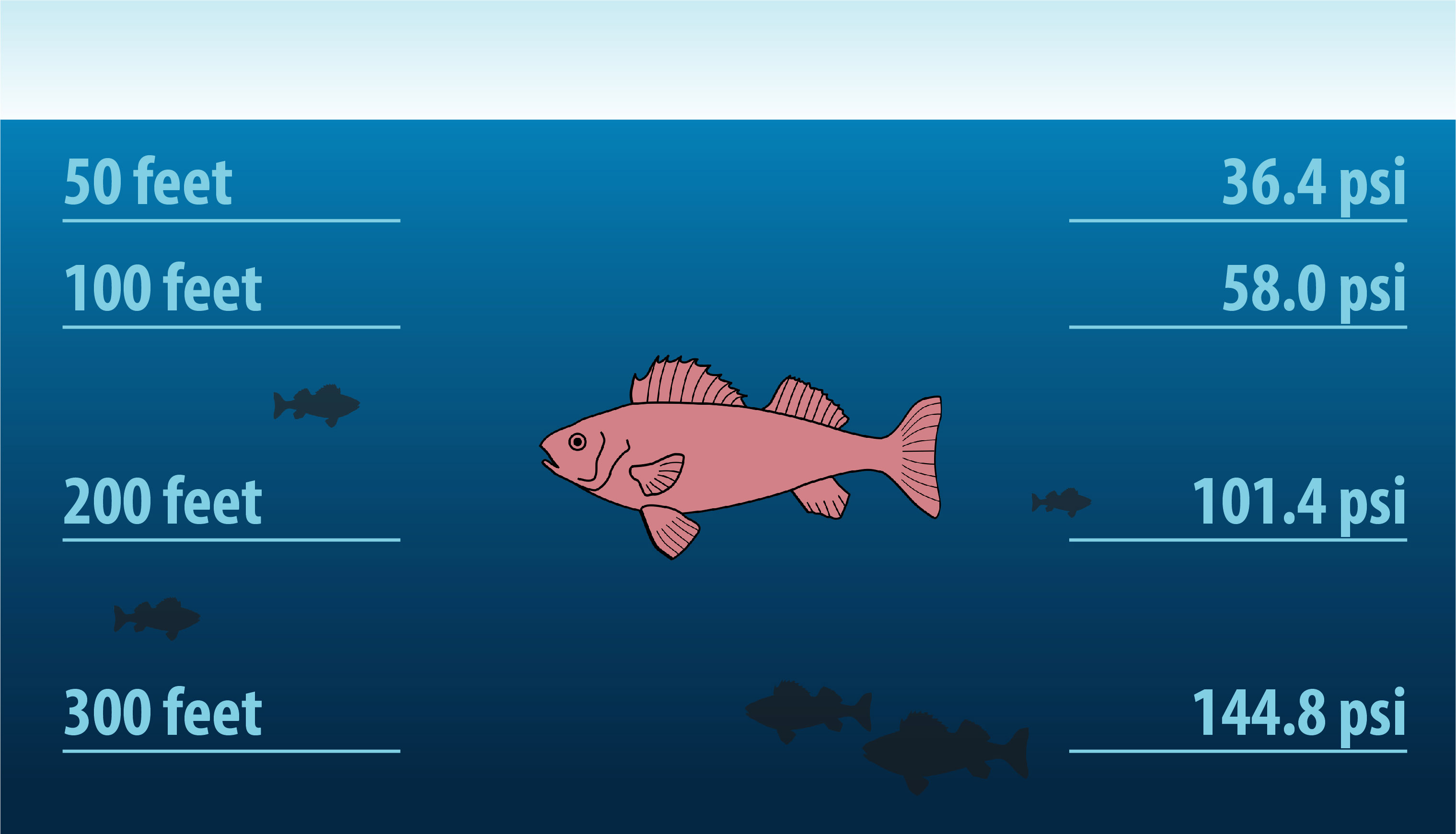
When you’re submerged in the ocean, you experience pressure from the force of the water molecules that surround you. At the surface, the pressure from the water is the same as the pressure from the air. Pressure increases the deeper you go in the ocean because there is more water above you. This water has weight, and weight is force. This force pushes against you, so you experience greater pressure all around.
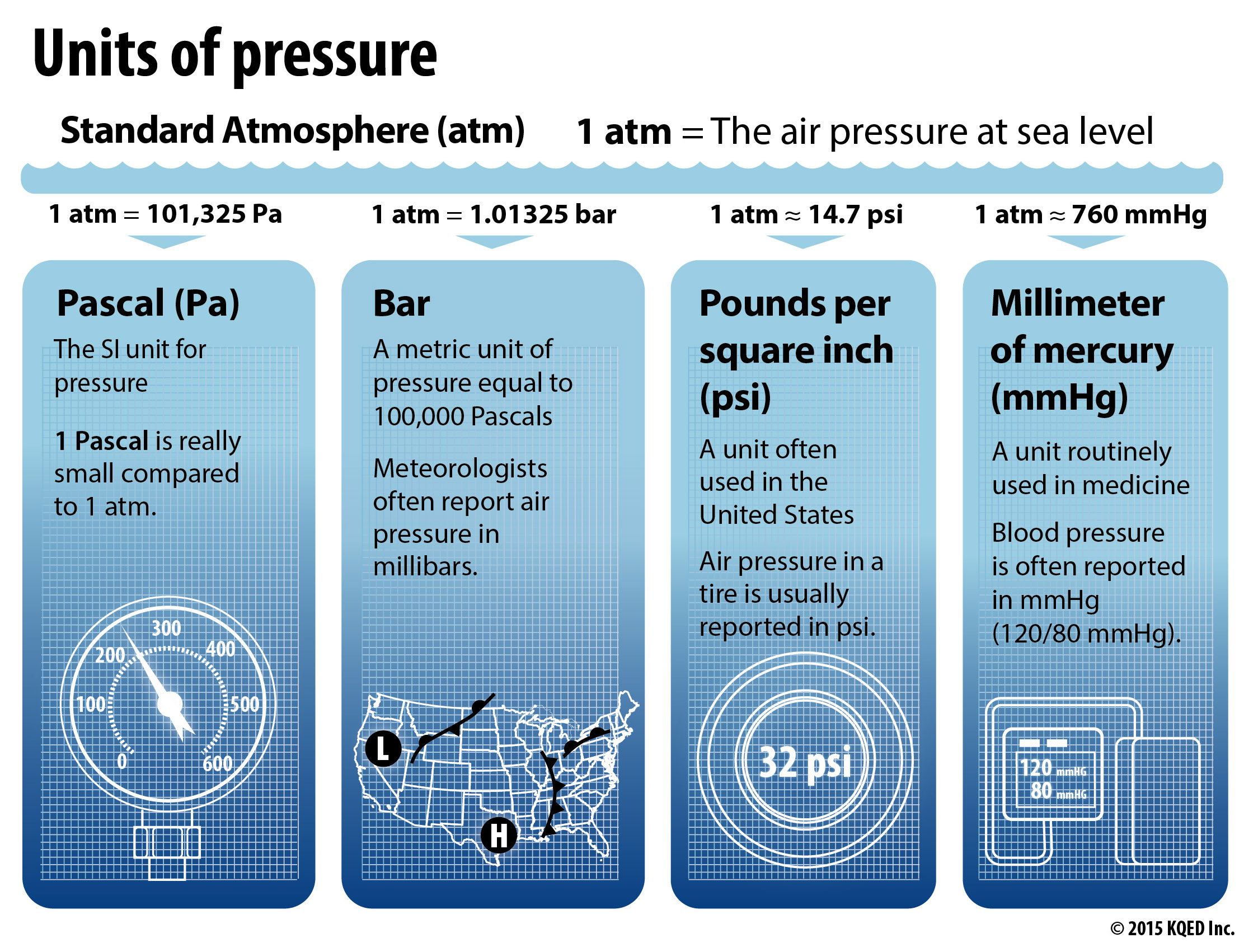
Pressure is measured in many different units. Air pressure at Earth’s surface is equal to one atmosphere (atm).
Why does this pressure matter for fish?
Many, but not all, fish have a gas-filled organ called a swim bladder. The swim bladder helps fish stay buoyant in the water. Without the swim bladder the density of their bodies would cause the fish to sink. The fish would have to keep swimming just to stay afloat. In fact, sharks don’t have swim bladders to keep them buoyant, so if they don't swim continually they sink to the bottom.
When a fish is quickly brought to the surface, the lower pressures allow the gas molecules inside the swim bladder to have more space to move. This causes the swim bladder to expand like a balloon. When the swim bladder expands, it presses on other organs. It can even force some organs out of the fish’s body. This often results in the death of the fish.
The relationship between volume and pressure is explained by Boyle’s law: As pressure increases, the volume of a gas decreases. As pressure decreases, the volume of a gas increases.
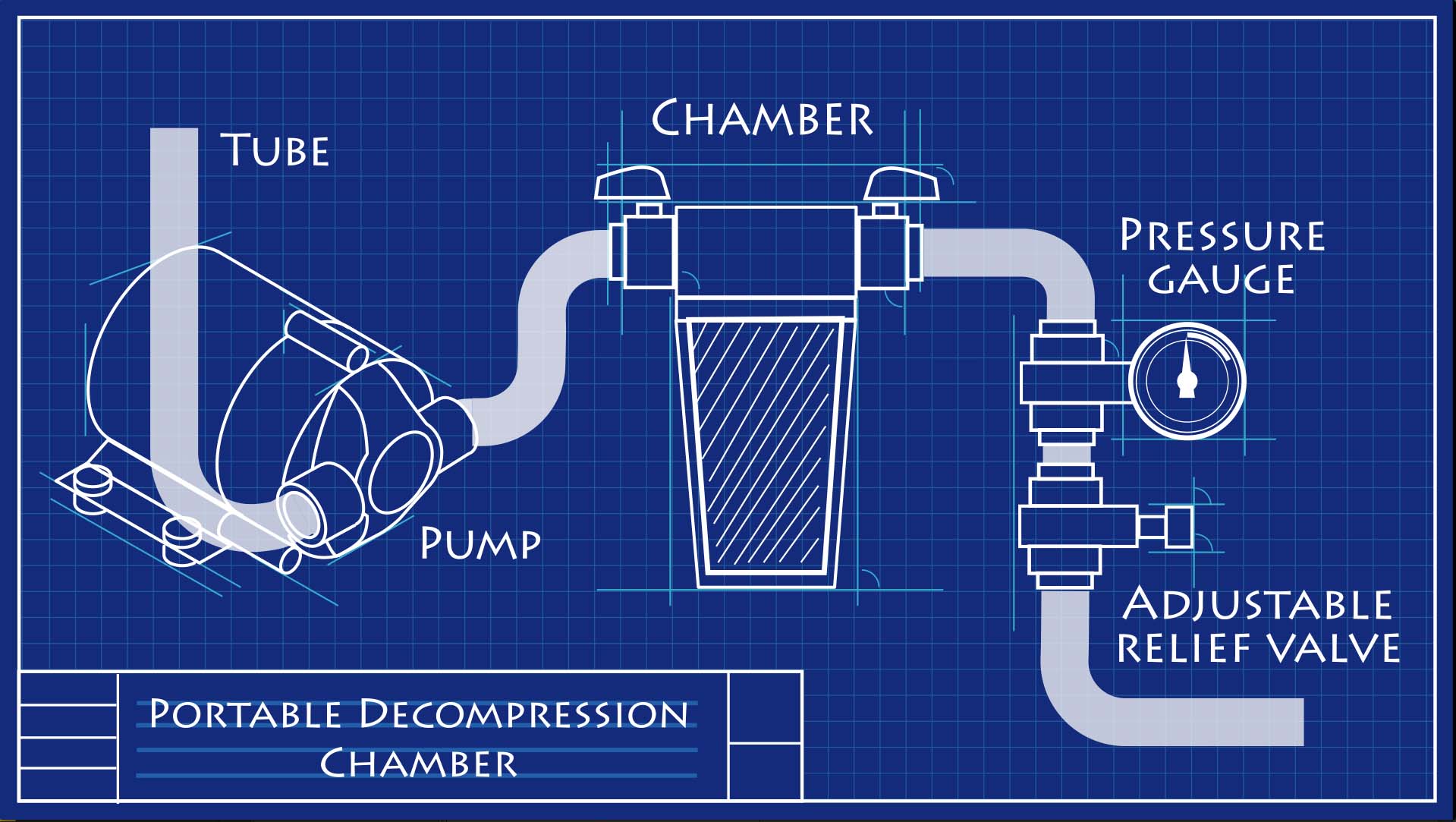
Matt Wandell uses a balloon and portable decompression chamber to illustrate what happens to a fish's swim bladder when it is subjected to changes in pressure. Having trouble viewing the video? Download or stream it on PBS Learning Media .
Scientists at the Academy engineered a portable decompression chamber that maintains high pressures while fish are brought to the surface. At the surface, the chamber is connected to a pump and valves that slowly decrease the pressure. Given enough time, many fish can regulate the gas in their swim bladders and adjust to slow changes in pressure. This is what the portable decompression chamber allows the fish to do.